

✓ Good Distribution Practice for Medical Device (GDPMD) ✓ Establishment License, Medical Device Authority (MDA) ✓ Laboratory Accreditation
(6 IN 1) SARS-COV-2 INFLUENZA A&B ANTIGEN, RSV, ADV AND MP COMBINED DETECTION KIT (LATEX METHOD)

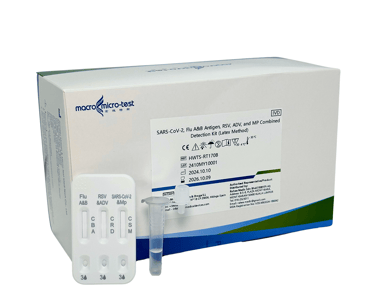
This kit is used for the in vitro qualitative detection of SARS-CoV-2, influenza A&B antigens, respiratory syncytial virus, adenovirus and mycoplasma pneumoniae in nasal swab, and nasopharyngeal swab samples, and can be used for the differential diagnosis of SARS-CoV-2 infection, respiratory syncytial virus infection, adenovirus infection, mycoplasma pneumoniae infection and influenza A or B virus infection. The test results are for clinical reference only and cannot be used as the sole basis for diagnosis and treatment.
(MDA Registration No: IVDC4863224-186042)
DENGUE NS1 ANTIGEN, IGM/IGG ANTIBODY DUAL
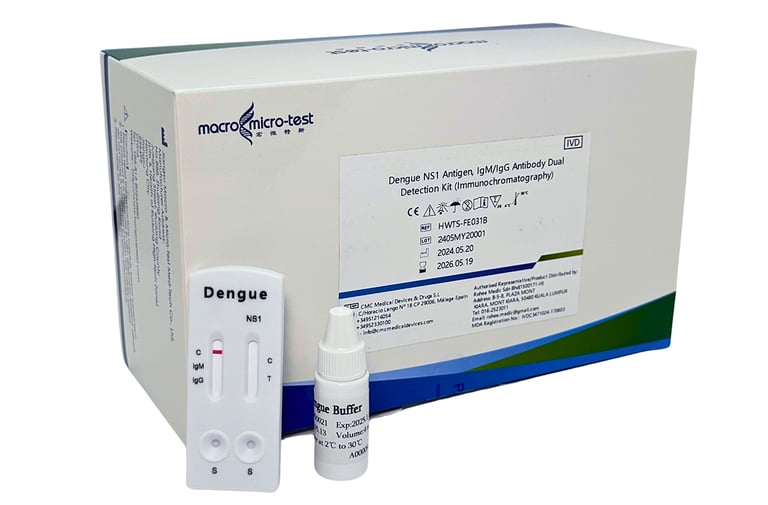
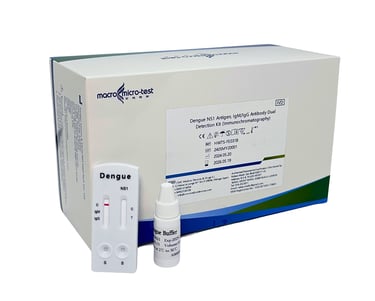
This kit is used for in vitro qualitative detection of dengue NS1 antigen and IgM/IgG antibody in serum, plasma and whole blood by immunochromatography, as an auxiliary diagnosis of dengue virus infection.
(MDA Registration No: IVDC3471024-170603)
SARS-COV-2 VIRUS ANTIGEN DETECTION KIT
(COLLOIDAL GOLD METHOD)-NASAL
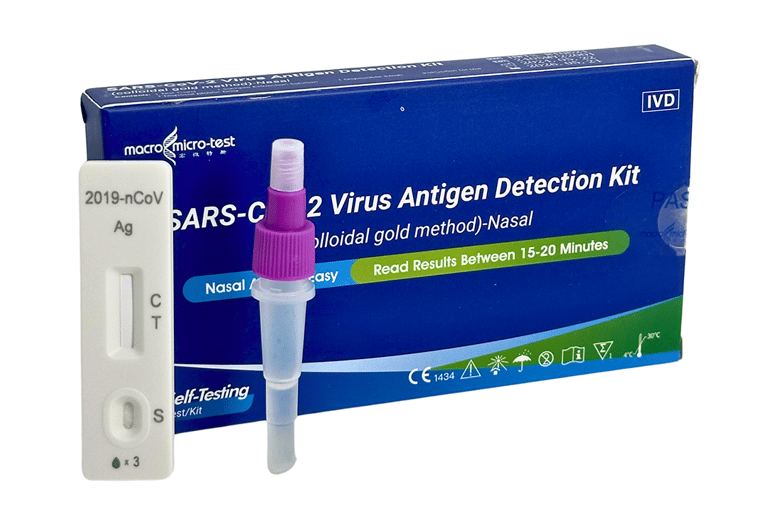
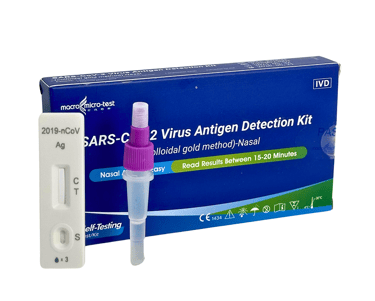
This Detection kit is for in vitro qualitative detection of SARS-CoV-2 antigen in nasal swab samples. This test is intended for non-prescription home use self-testing with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older who are suspected of COVID-19 or adult collected nasal swab samples from individuals under 15 years old who are suspected of COVID-19.


