

✓ Good Distribution Practice for Medical Device (GDPMD) ✓ Establishment License, Medical Device Authority (MDA) ✓ Laboratory Accreditation
LYHER COVID-19 AND INFLUENZA A/B ANTIGEN TEST KIT
(MDA Registration No: IVDC3966223-146670)
Evaluated by Institute for Medical Research (IMR) shows that it is highly accurate with: Sensitivity : 96.7%, Specificity : 100%
The LYHER® COVID-19 and Influenza A/B Antigen Test Kit is an in vitro immunoassay. The assay is for the direct and qualitative detection of antigen of SARS-CoV-2 Influenza A/B from nasopharyngeal secretions and oropharyngeal secretions. The kit is for in vitro diagnostic use.
- CE Marked
- Results in 15mins
- Easy to read results
- Easy to collect specimen at just 2-3.5cm depth
- Room temperature storage

LYHER INFLUENZA A/B ANTIGEN TEST KIT (COLLOIDAL GOLD)
(MDA Registration No: IVDC3489323-140142)
The LYHER®InfluenzaA/B Antigen Test Kit is an in vitro immunoassay. The assay is for the direct and qualitative detection of antigen of Influenza A/B from nasopharyngeal secretions and or opharyngeal secretions. The kit is for in vitro diagnostic use.
- CE Marked
- Results in 15mins
- Easy to read results
- Easy to collect specimen at just 2-3.5cm depth
- Room temperature storage

LYHER PREGNANCY
(HCG) TEST KIT - STRIP

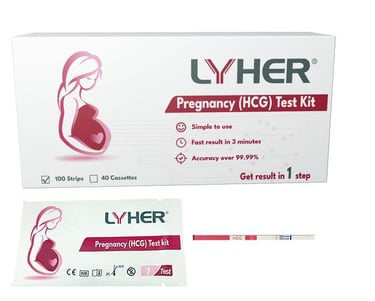
Lyher Pregnancy (HCG - human chorionic gonadotropin) Test Kit is a highly sensitive test kit. From early detection to efficient results, this diagnostic solution is tailored to meet the demands of modern healthcare.
(MDA Registration No: IVDB8139924-160553)
LYHER DENGUE NS1/IgG/IgM
TEST KIT
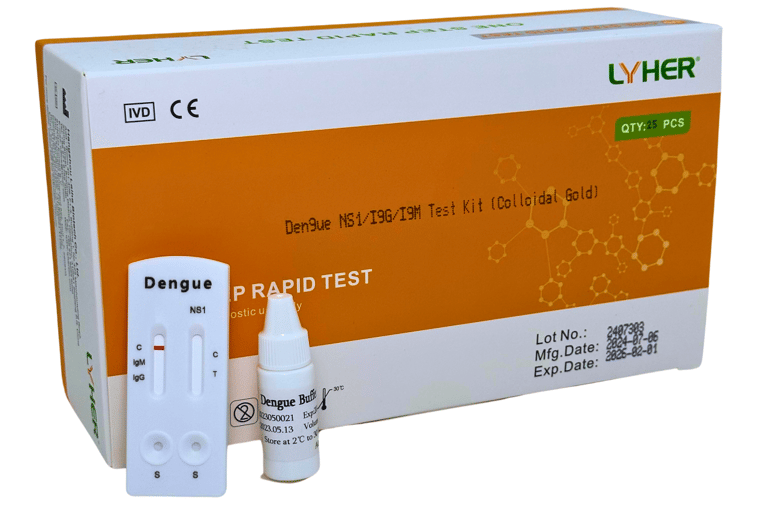
Lyher Dengue Test Kit is a rapid chromatographic immunoassay for the qualitative The Dengue detection of dengue virus NS1 antigen and IgM/ IgG antibodies to dengue virus in whole blood, serum or plasma ComboRapidTest to aid in the diagnosis of dengue viral infection.
(MDA Registration No: IVDC7782224-164625)
LYHER PREGNANCY
(HCG) TEST KIT - MIDSTREAM
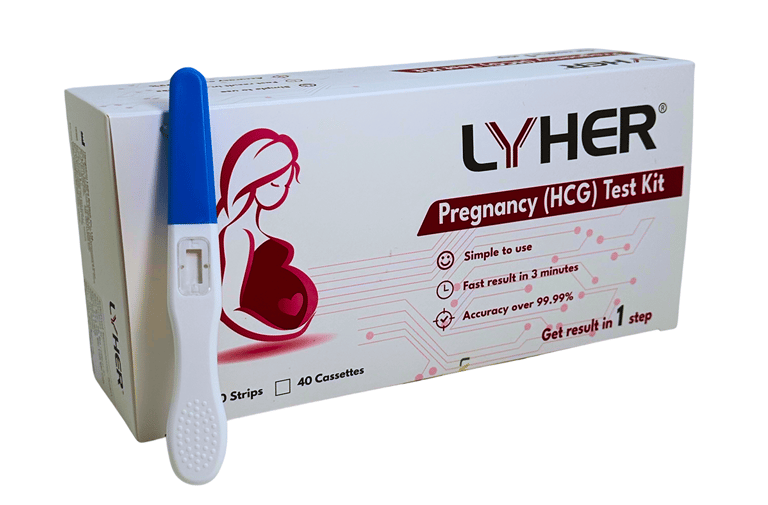
Lyher Pregnancy (HCG - human chorionic gonadotropin) Midstream Test Kit is a highly sensitive test kit. From early detection to efficient results, this diagnostic solution is tailored to meet the demands of modern healthcare.


